Enhanced Flexible Mold Lifetime for Roll‐to‐Roll Scaled‐Up Manufacturing of Adhesive Complex Microstructures
Bioinspired Microstructured Adhesives with Facile and Fast Switchability for Part Manipulation in Dry and Wet Conditions
Smart Materials for manipulation and actuation of small-scale structures
3D nanofabrication of various materials for advanced multifunctional microrobots
Liquid Crystal Mesophase of Supercooled Liquid Gallium And Eutectic Gallium–Indium
Machine Learning-Based Pull-off and Shear Optimal Adhesive Microstructures
Information entropy to detect order in self-organizing systems
Individual and collective manipulation of multifunctional bimodal droplets in three dimensions
Microrobot collectives with reconfigurable morphologies and functions
Self-organization in heterogeneous and non-reciprocal regime
Biomimetic Emulsion Systems
Giant Unilamellar Vesicles for Designing Cell-like Microrobots
Bioinspired self-assembled colloidal collectives drifting in three dimensions underwater
Immune cell-based microrobots for targeted cancer therapy
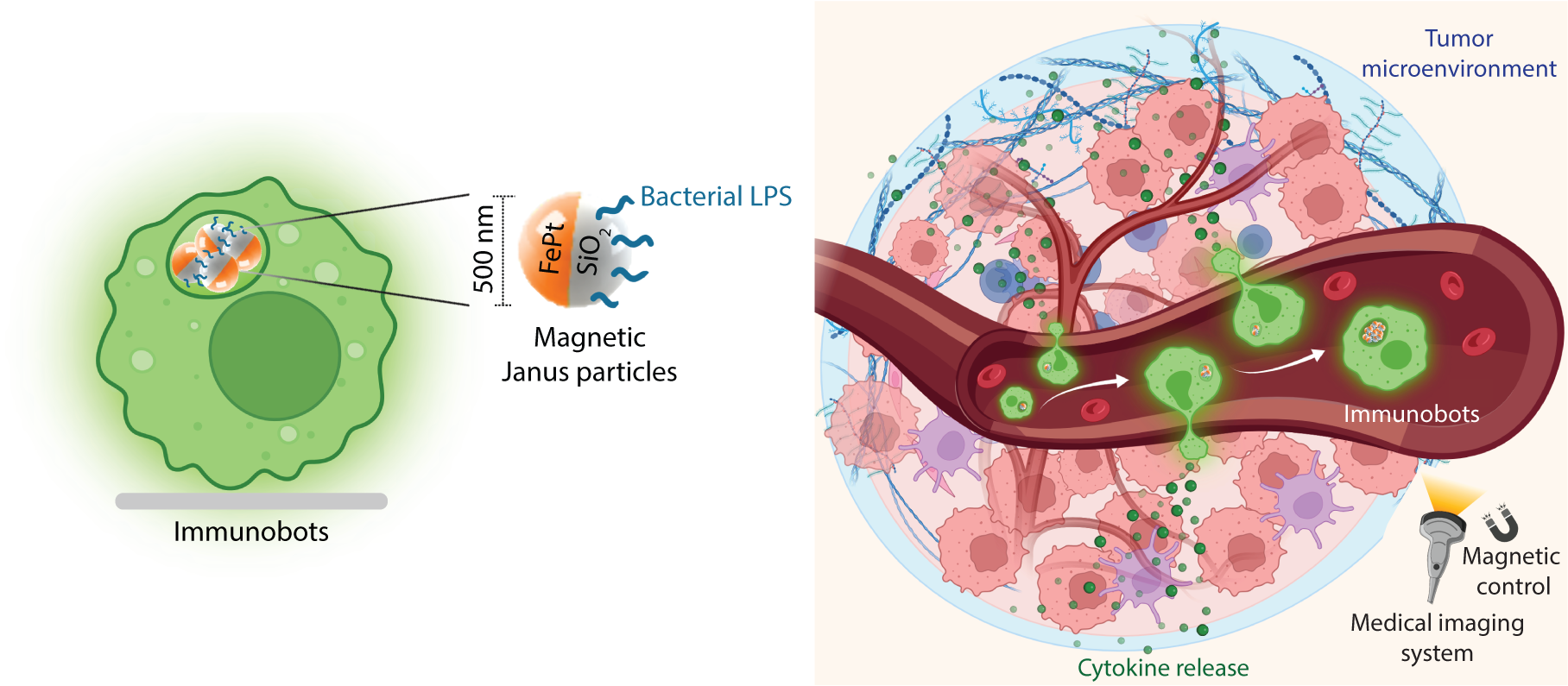
Building medical microrobots from the body's own cells may circumvent the biocompatibility concern and hence present more potential in clinical applications to improve the possibility of escaping from the host defense mechanism. More importantly, live cells can enable therapeutically relevant functions significantly more efficiently than synthetic systems. In this direction, living immune cell-derived microrobots from macrophages, immunobots, which can be remotely steered with externally applied magnetic fields and directed toward anti-tumorigenic (M1) phenotypes, were presented [1]. Macrophages engulfed the engineered magnetic nanoparticles, composed of 500 nm diameter silica Janus particles with one side coated with FePt magnetic nanofilm for magnetic steering and medical imaging and bacterial lipopolysaccharides for stimulating macrophages in a tumor-killing state. The torque-based surface rolling locomotion of the immunobots was demonstrated inside blood plasma, over a layer of endothelial cells, and under physiologically relevant flow rates. The immunobots secreted signature M1 cytokines and M1 cell markers via stimulation with bacterial lipopolysaccharides. The immunobots exhibited anticancer activity against urinary bladder cancer cells. Such immunobots were further developed from freshly isolated primary bone marrow-derived macrophages since patient-derivable macrophages have strong clinical potential for future cell therapies in cancer while eliminating side effects and unwanted immune reactions [1].
Next, the immunobots were further investigated using medical imaging modalities, such as magnetic resonance imaging and optoacoustic imaging in soft-tissue-mimicking phantoms and ex vivo conditions. Magnetic actuation and real-time imaging of immunobots were demonstrated under static and physiologically relevant flow conditions using optoacoustic imaging [2]. The proposed approach demonstrated the proof-of-concept feasibility of integrating macrophage-based microrobots into clinic imaging modalities for cancer targeting and intervention, and can also be implemented for various other medical applications.
Members
Publications







